Abstract
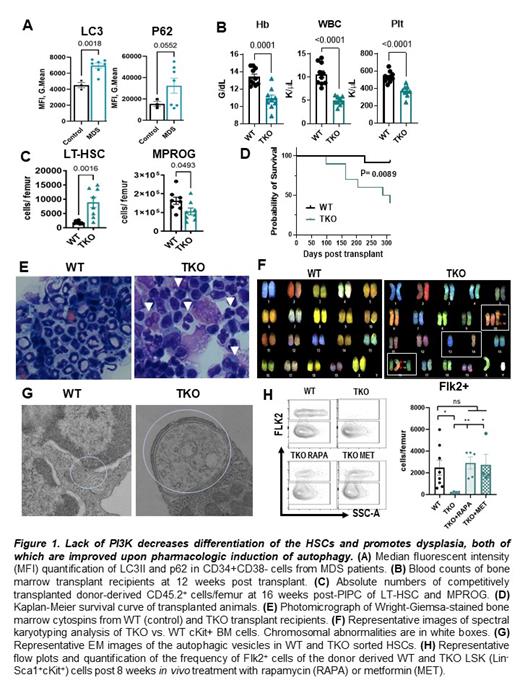
Myelodysplastic Syndrome (MDS) is a heterogeneous clonal malignancy arising in hematopoietic stem cells (HSCs), characterized by ineffective hematopoiesis, cytopenias, and the potential to progress to acute myeloid leukemia (AML). However, the perturbations in HSCs that lead to MDS initiation are poorly understood. It has been reported that HSCs are particularly dependent on autophagy for the maintenance of differentiation and self-renewal. We observed that, compared to healthy donor bone marrow hematopoietic stem and progenitor cells (HSPCs), MDS patient stem and progenitor cells (Lin-CD33-CD34+CD38-) have abnormal levels of autophagic degradation, as demonstrated by abnormal intracellular LC3II and P62 staining (Figure 1A). Autophagy is known to be regulated by the (PI3K)/AKT pathway, which transduces hematopoietic growth factor and cytokine signals in HSCs. PI3K/AKT is frequently activated in AML, but its role in MDS is less clear. Surprisingly, we found that CD34+ cells from a subset of MDS patients have upregulated expression of PTEN, the major negative regulator of the PI3K/AKT pathway, suggesting that PI3K/AKT may be downregulated in MDS stem cells. Therefore, we hypothesized that the Class IA PI3K isoforms (P110α, β, and δ) are required to maintain HSC differentiation and self-renewal.
To understand the consequences of PI3K downregulation in HSCs, we generated a triple knockout (TKO) mouse model with conditional deletion of P110α and P110β in hematopoietic cells, and germline deletion of P110δ. Surprisingly, we found that PI3K deletion causes transplantable pancytopenia and decreased survival, despite the abnormal expansion of donor TKO HSCs (Figure 1 B,C). Consistent with this inefficient hematopoiesis, TKO bone marrow cells exhibited dysplastic features in multiple blood lineages and multiple chromosomal abnormalities (Figure 1 E,F), suggesting that PI3K inactivation in HSCs can promote MDS initiation. To determine whether impaired HSC differentiation in TKO mice could be due to dysregulated autophagy, we assessed autophagy in TKO HSCs by flow cytometry and immunofluorescence with the autophagosomal marker, LC3II. Our results showed that, compared to the WT controls, TKO HSCs have inefficient autophagic flux and decreased degradation of the cargo protein P62. We also discovered that TKO HSCs have significantly enlarged autophagic vesicles (Figure 1 G), and impaired fusion of autophagosomes with lysosomes, consistent with a marked defect in autophagic degradation. Treatment of TKO mice with two pharmacologic inducers of autophagy, rapamycin or metformin, improved HSC differentiation with an increase in Flk2+ MPPs (Figure 1 H), reduced dysplasia, and decreased the size of the TKO mutant clone in chimeric mice. Thus, our results uncover an important role for PI3K in regulating autophagy in HSCs to maintain the proper balance between self-renewal and differentiation. Our new mouse model of MDS will be a useful tool to study the mechanisms of MDs initiation. In addition, our findings open exciting avenues for future investigations of autophagy-inducing agents in MDS.
Verma: Celgene: Consultancy; Stelexis: Current equity holder in publicly-traded company; Throws Exception: Current equity holder in publicly-traded company; Acceleron: Consultancy; Novartis: Consultancy; Stelexis: Consultancy, Current equity holder in publicly-traded company; Eli Lilly: Research Funding; Curis: Research Funding; Medpacto: Research Funding; Incyte: Research Funding; BMS: Research Funding; GSK: Research Funding. Gritsman: iOnctura: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal